Unit 31: Kinetic
Molecular Theory
Unit Overview:
Gases
are all around us and we use and produce gases all the time. In this unit, we
will take a conceptual view of gases. This will allow us visualize how gases
behave and their relationship to pressure, temperature, and volume changes.
In
this unit, you will complete a visualization exercise using PhET simulation on
Gas Properties.
Kinetic
Molecular Theory
The
experimental observations about the behavior of gases discussed so far can be
explained with a simple theoretical model known as the kinetic molecular
theory. This theory is based on the following postulates, or assumptions.
|
POSTULATE 1 |
Gases are very small
molecules and their size is insignificant compared to the space around them. |
|
POSTULATE 2 |
Gas molecules move in straight lines at different
speeds. |
|
POSTULATE 3 |
The intermolecular force between gas molecules is negligible, except
at the moment they collide. |
|
POSTULATE 4 |
When gas molecules collide, their collisions are
elastic. |
|
POSTULATE 5 |
The average kinetic energy is directly related to the absolute
temperature. |
The kinetic
molecular theory (KMT) is a simple microscopic model that effectively
explains the gas laws. This theory is based on the following five
postulates above. The term “molecule”
will be used to refer to the individual chemical species that compose the gas,
although some gases are composed of atomic species, for example, the noble
gases.
The
kinetic molecular theory of gases describes this state of matter as composed of
tiny particles in constant motion with a lot of distance between the
particles. Because most of the volume occupied by a gas is empty space, a
gas has a low density and can expand or contract under the appropriate
influence. The fact that gas particles are in constant motion means that two or
more gases will always mix as the particles from the individual gases move and
collide with each other. The number of collisions the gas particles make with
the walls of their container and the force with which they collide
determine the magnitude of the gas pressure.
Watch the video below:
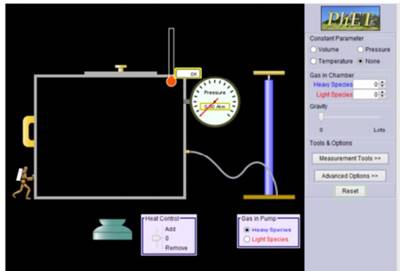
Simulation: Gas Properties
Click the picture to run the
simulation.
You will conduct the Gas Properties Simulation for
Postulates 1 – 5. Please complete the Student Exploration Sheet while conducting the simulation.
|
Postulate 1 Gases
are very small molecules and their size is insignificant compared to the
space around them. |
|
When you open the
simulation, you should not see any gases in the box. If you do, click Reset.
Your goal in this section is to determine what postulate 1 means. |
|
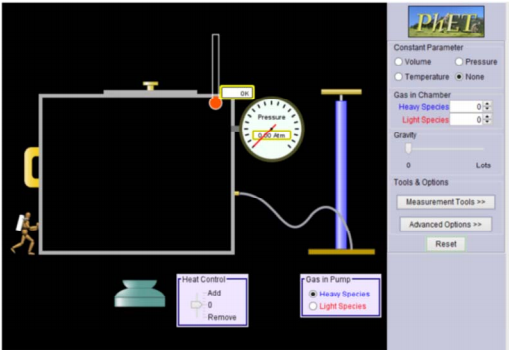
1. Set your beginning
parameters as shown below.
|
|
2. Lift the tire pump
all the way up and give it one pump down. |
|
3. Looking at what is
happening inside the box, explain what would happen to a person in the box
who was trying to walk from left to right. |
|
4. Now consider
postulate 1, Gases are very small molecules and their size is insignificant
compared to the space around them. Does this make sense given your
description of what would happen to the person? Explain. |
|
5. If you had to
design the simulation so that postulate 1 would be very easy to see, what
would you change? (Hint: Why is it that we as humans can walk outside and not
feel the constant bombardment of gaseous oxygen and nitrogen molecules from
air, hitting our bodies?) |
|
Postulate 2 Gas
molecules move in straight lines at different speeds. |
|
1. Continue to use
the same gas molecules in the box as you did for postulate 1. (In other
words, don’t add any more gas molecules yet, or changes any parameters.) |
|
2. Look at the motion
of the particles. Are they moving in straight lines and do they appear to be
moving a different speed? Why do you think gas particles move at different
speeds? |
|
3. Reset the
simulation. Move the tire pump handle very, very, little. Your goal is to try
and get only gas particle in the box. Watch the motion of this gas particle.
Does its speed change as it hits the walls of the container? Does it ever
curve? |
|
4. Just for fun,
increase or lower the temperature and see if this makes the gas particles
curve. Try to predict what is going to happen prior to changing the
temperature. To change the temperature, move the slider on the heat control
up or down. |
|
5. Now add one more
gas molecule. If you are lucky, they will hit each other. When they collide,
can you tell if there is a difference in speed? Do they continue to move in
straight lines? |
|
Postulate 3 The
intermolecular force between gas molecules is negligible, except at the
moment they collide. |
|
1.
First off, remember that an intermolecular force
is something that attracts molecules to one another. |
|
2.
Put at between 10 and 20 gas molecules into your
box. |
|
3.
Follow the motion of just one gas molecule for the
moment. At any point in time, except when it is hitting another molecule or
the walls of the container, does it appear to be attracted to other
molecules? Describe why or why not. Be specific in your description. |
|
4.
I think you will see that this postulate is very
much related to postulate 2, in that the straight-line travel of gas
particles does happen, because molecules are not attracted to one another. |
|
When gas molecules collide, their
collisions are elastic. |
|
1. What does elastic mean? Many of us think of the elastic waist band
on our pants, sweat pants, or those crazy stretch pants that athletes often
wear. Answer the following question….What makes something elastic? |
|
2. Now let’s approach this from a different angle,
what would happen if you were wearing elastic pants and they stopped being
elastic? |
|
3. Now that we know what happens when something isn’t elastic, let’s
relate it to the gas molecules bouncing around on our computer screen. What
would happen if the collisions were not elastic? |
|
4. In the kinetic
molecular theory, unlike with a bungee cord drop, these collisions are
elastic and transfer 100% energy. The overall energy stays constant, but each
particle can change and have different energies after each collision. |
|
5. For fun, see if
you can vary the volume, temperature, or pressure and make the collisions
inelastic. |
|
Postulate 5 The
average kinetic energy is directly related to the absolute temperature. |
|
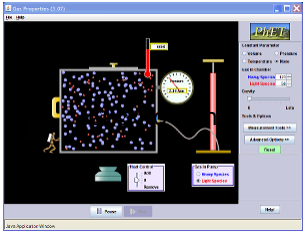
1.
Put 30 or more molecules in the box |
|
2.
Increase the heat by moving the heat control slide
to add. What happens to speed of the molecules? |
|
3.
Click on measurement tools |
|
4.
Click on energy histograms. |
|
5. Observe the top
graph which shows the kinetic energy of the particles. Describe what happens
to the energy. What is meant by average kinetic energy as you look at this
graph? |
|
6. Now cool the
particles. Does the average kinetic energy increase or decrease? |
|
7. Grab the handle on
the left side of the box and push it slowly to the right. Here, you are decreasing
the volume. What happens if you decrease the volume too far? |